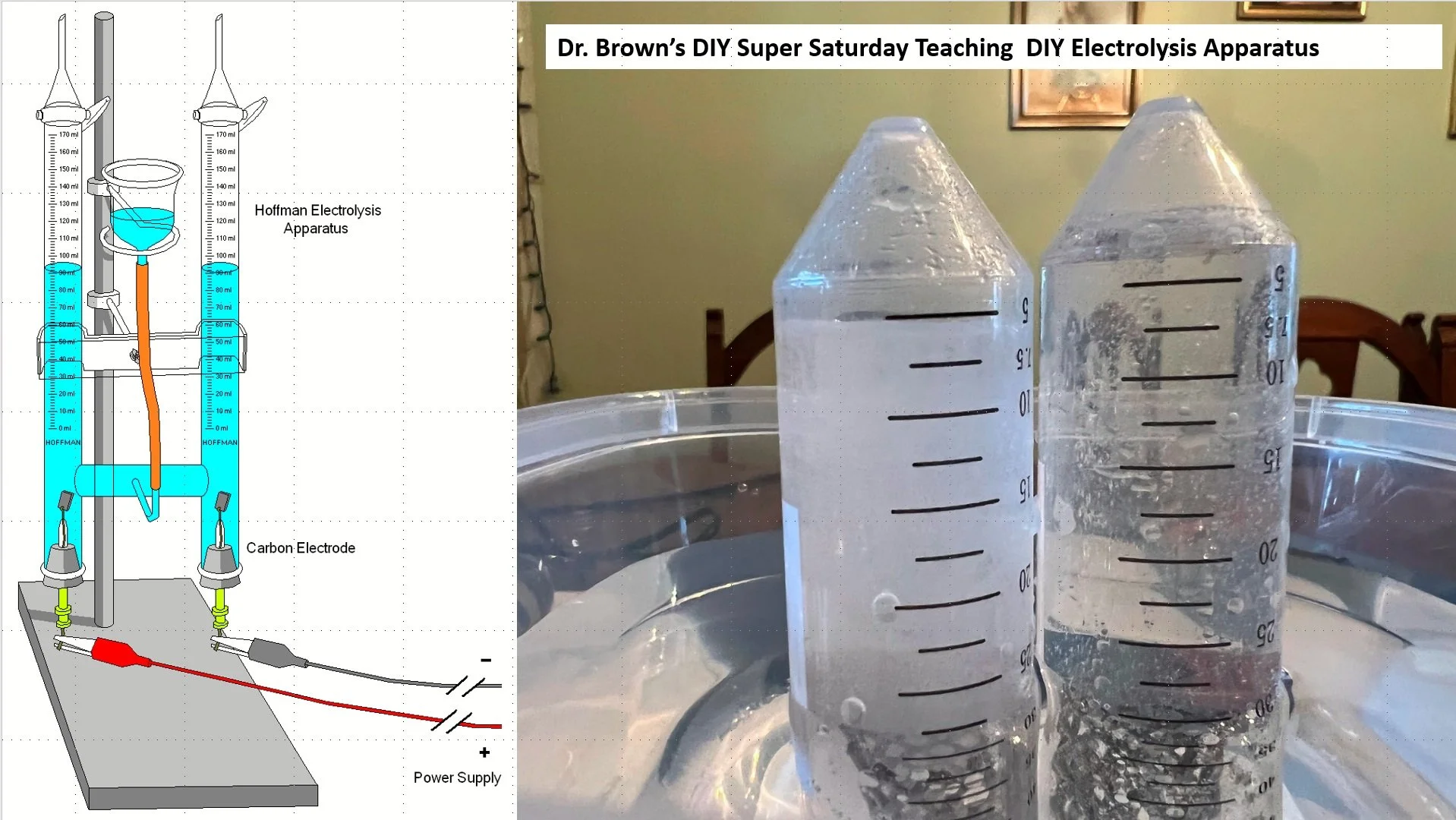
DIY Hoffmann Apparatus
To evaluate and compare the Hoffman Apparatus, we will focus on two distinct versions: the commercially available model, often found through a simple internet search, and Dr. Brown's DIY Hoffman Apparatus, created as part of the Super Saturday Program. The commercial version typically ranges in price from $50 to $300.
First, let's delve into the commercial Hoffman Apparatus. This apparatus, a staple in chemical laboratories, is designed for the electrolysis of water, a fundamental experiment in understanding chemical reactions and electrical conductivity. The apparatus consists of two vertical glass tubes connected by a horizontal tube, allowing for the observation of gas evolution at the anodes and cathodes during electrolysis.
In contrast, Dr. Brown's DIY Hoffman Apparatus, documented on the naturalbornscientists.com website, exemplifies the integration of hands-on learning and cost-effectiveness in educational settings. This version, created with budget-friendly materials, provides an accessible way for students to engage with complex scientific concepts. Building such an apparatus not only deepens the understanding of the underlying principles of electrolysis but also enhances skills in problem-solving and creativity.
The DIY approach, particularly in a program for gifted students, fosters a deeper appreciation for the practical aspects of scientific inquiry. It demonstrates the feasibility of conducting significant experiments with limited resources, thus encouraging innovation and adaptability.
In conclusion, while the commercial Hoffman Apparatus offers a ready-to-use solution for studying electrolysis, Dr. Brown's DIY version presents a unique opportunity for students to immerse themselves in the practical aspects of scientific construction and experimentation. Both versions serve the essential purpose of elucidating the principles of electrolysis, yet the DIY apparatus adds an invaluable dimension of hands-on learning and ingenuity.
.
Hofmann Voltameter: An Instrument in Elucidating Atomic Theory
August Wilhelm von Hofmann was born on April 8th 1818 in Giessen, Germany years after Proust published the Law of Definite proportions.
The Hofmann Voltameter holds a specialized position in the history of atomic theory by providing empirical evidence for the nature of atoms and molecules. Designed to facilitate the electrolysis of water, the apparatus demonstrates that water disassociates into two hydrogen atoms for every oxygen atom. This 2:1 ratio solidifies Avogadro's hypothesis, which postulates that equal volumes of gases, at the same temperature and pressure, contain an equal number of molecules.
Through the quantitative analysis enabled by the Hofmann Voltameter, scientists could reliably interpret the stoichiometric relationships between elements. The produced hydrogen and oxygen gases not only adhere to a fixed volumetric ratio but also conform to defined mass ratios. Such observations lend credence to the tenets of atomic theory that emphasize the conservation of mass and the discrete nature of atoms.
Furthermore, the voltameter facilitates the study of Faraday's laws of electrolysis, strengthening our grasp on the interaction between electric currents and atomic structures. By quantifying how much charge is needed to disassociate a certain amount of substance, it makes an unambiguous connection between electrical energy and atomic transitions.
In summary, the Hofmann Voltameter functions as an essential experimental tool that offers pivotal insights into atomic behavior and relationships. Its contributions extend beyond simple electrolysis, serving as an instrumental piece in understanding the intricacies of atomic theory. Today, the Hofmann apparatus can be found as a teaching tool in the finest of universities throughout the world. However, it would be an insult to
this man,s legacy not to mention that he had a pleura of other contributions to science, also.
Joseph Proust and the Law of Definite Proportions
Joseph Proust's seminal work in chemistry, culminating in the early 19th century, led to the establishment of the Law of Definite Proportions, a cornerstone in the field of chemical analysis and composition. Proust's law fundamentally asserts that chemical compounds are always composed of elements in constant proportions by mass. This principle, also known as Proust's Law, challenged the prevailing theory of variable proportions and significantly advanced the development of atomic theory.
Proust's defining experiments were not limited to, but notably included, the analysis of copper carbonate. Through his meticulous approach, he heated and decomposed copper carbonate (CuCO3) into copper oxide (CuO) and carbon dioxide (CO2), as represented by the chemical equation CuCO3 → CuO + CO2. Proust observed that regardless of the source or the amount of copper carbonate used, the ratio of copper to carbon to oxygen in the compound remained constant. This consistent ratio led to the formulation of his Law of Definite Proportions: a chemical compound always contains exactly the same proportion of elements by mass.
The timeline of Proust's research is crucial for understanding the development of this law. While some of his early work began around 1794, his most significant contributions and publications that solidified the Law of Definite Proportions occurred between 1797 and 1804. It is during this period that he refined his theory and presented compelling empirical evidence to support it.
Proust's work was pivotal in shifting the scientific understanding of chemical compounds from the previously held belief in variable proportions to a recognition of fixed compositional ratios. This shift laid the groundwork for the atomic theory of matter, influencing future scientific research and education, particularly in atomic theory.
Joseph Proust’s meticulous experimental approach and the discovery of the Law of Definite Proportions exemplify the awe and wonder inherent in scientific discovery. His contributions have inspired generations of chemists and students in the field of chemical sciences, marking a monumental moment in the history of chemistry.
Representation of Lavoisers’ famous Las of Conservation of Mass experiment
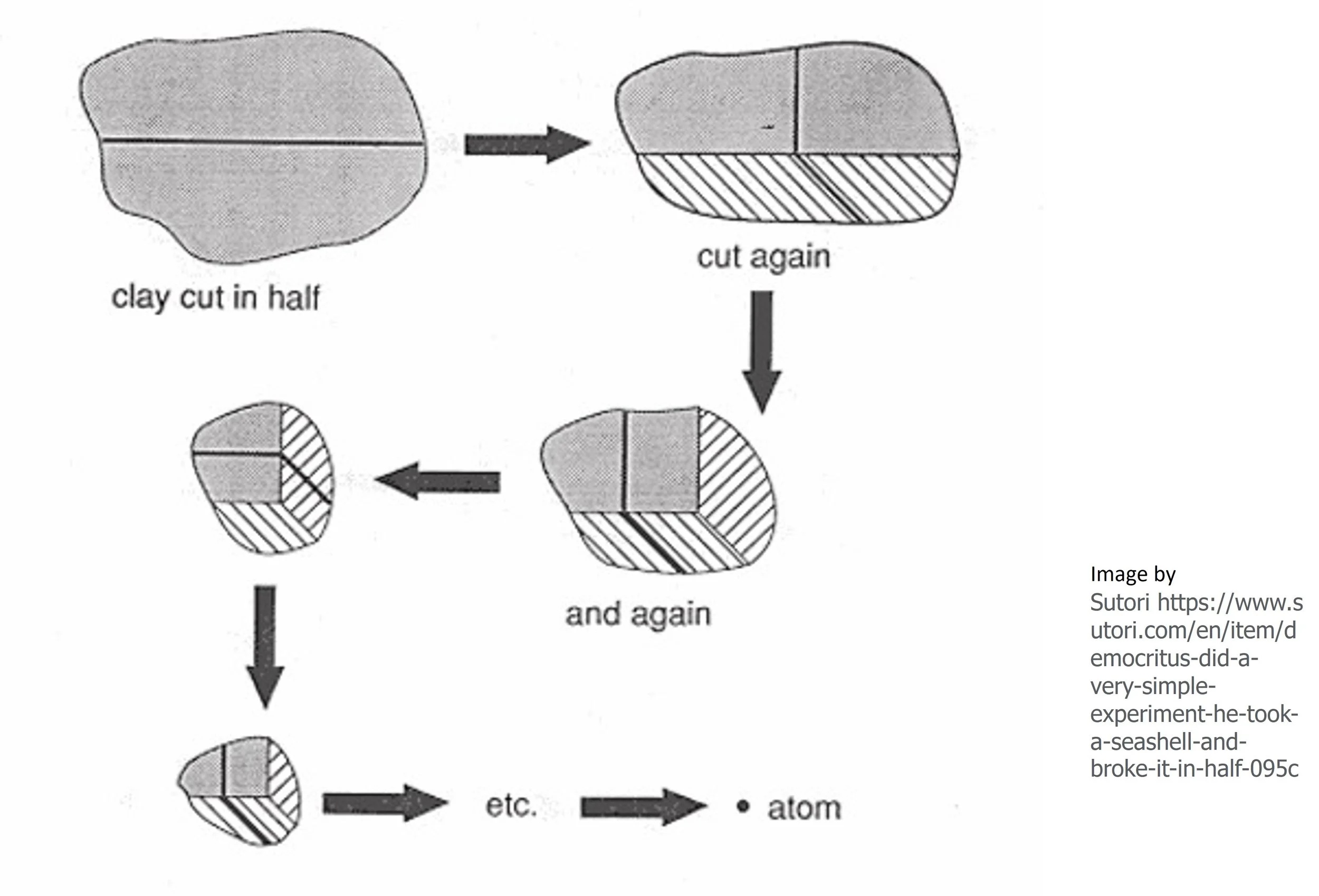
Ancient Atomic Theory and Lavoisier's’ Conservation of Mass Experiment
The insightful philosophers Leucippus and Democritus unveiled a hypothesis, a guess, about matter that has captivated thinkers for centuries. They postulated that:
1.The Indivisible: Everything is made of tiny, eternal particles called "atomos" — uncuttable.
2. The Void: These atoms move in a space, termed the "void."
3. Collisions and Alliances: Atoms encounter each other, sometimes combining or bouncing apart upon impact.
4. Diversity of Forms: Different substances are composed of atoms with varied sizes and shapes.
5. Qualitative Essence: Atoms’ unique arrangements and forms grant distinct sensory qualities to matter.
Centuries later, the Lavoisier partnership, through their pioneering experiment, provided evidence of these principles. "Lavoisier was a Parisian through and through and a child of the enlightenment," wrote biographer Henry Guerlac. Their Law of Conservation of Mass experiment presents empirical data mainly supporting ancient postulates 1 and 3: atoms are uncuttable, and they collide and bond without loss or gain in mass. By demonstrating that water is a compound formed from oxygen and hydrogen, not a singular element, they showed that atoms retain their identity through chemical reactions. The total mass remains constant, whether the atoms are free or combined, an enduring proof of the law and a brilliant affirmation of the atomic theory envisioned by the ancients. This revelatory work, highlighting Marie and Antoine Lavoisier's contributions, bridges the gap between ancient philosophical musings and empirical scientific evidence and continues to spark awe and inspiration in scientific exploration.
In June 1783, Lavoisier reacted oxygen with inflammable air, obtaining "water in a very pure state." He correctly concluded that water was not an element but a compound of oxygen and inflammable air, or hydrogen as it is now known. To support his claim, Lavoisier decomposed water into oxygen and inflammable air. Now, the composition of water was known. The experiment was published as Traité élémentaire de Chimie (Elements of Chemistry) in Paris in 1789.
The Lavoiser’s Discover Another Element of Chemistry, Love
Meet Antoine and Marie Anne Lavoisier, the power couple behind groundbreaking chemical discoveries. Imagine a world where every time you mix things together, they always combine in the same way, like a perfect recipe. That's the world the Lavoisiers helped us understand. They discovered that substances always mix in exact amounts and that nothing gets lost or magically appears in reactions—a big deal called the conservation of mass!
But their story isn't just about science. It's also about sticking together through thick and thin. When the world turned upside down during the French Revolution, Marie-Anne kept their scientific dream alive, even after Antoine's life was taken too soon. She didn't just sit back; she made sure the world knew about the amazing things they discovered together.
Marie-Anne and Antoine weren’t just husband and wife; they were science buddies, proving that teamwork can lead to amazing discoveries. Their story shows us that with passion and support, we can overcome challenges and make a lasting impact—just like they did with their love for science and each other.
Let's dive into a chemistry mystery that the Lavoisier's helped solve! Have you ever wondered what makes things burn? Antoine Lavoisier discovered it's something in the air called oxygen. When things burn, they actually combine with oxygen. It's like a dance where oxygen partners up with other elements to create something new—this is combustion.
Robert Boyle: The First Modern Chemist, 1627
Imagine being a young noble, Robert Boyle, whose childhood curiosity within the vast halls of an Irish castle sparked a lifelong quest for knowledge. His education at Eton College wasn't merely academic; it was a quest to decipher the secrets of nature. Boyle's journey for understanding took him across the vibrant landscapes of France and Italy, where he immersed himself in the language to grasp Galileo's works directly.
Though Boyle never met Galileo, he inherited the great scientist's inquisitive spirit. In Ireland, he converted his inheritance into a laboratory for experimentation, leading to the groundbreaking Boyle's Law on gases—a principle pivotal to our understanding of chemistry.
Inspired by Boyle's relentless curiosity, Natural Born Scientists, LLC encourages you to emulate this trailblazer. They provide accessible science projects that let you become the detective, unraveling the wonders uncovered by preceding scientists. They champion the transformation of simple materials into remarkable revelations, echoing the alchemical dreams of turning base metals into gold.
With their resources, you can dive into experimentation, potentially leading to novel discoveries that could echo Boyle's impactful experiments. It's a call to arms—a reminder that the thirst for discovery is eternal and awaits anyone ready to don a lab coat and embark on this timeless adventure.
Visit "DIY Boyle’s Law Apparatus" on their webpage for a hands-on experience of Boyle's enduring legacy.